Earlier Extraction for Cardiac Device Infections Tied to Lower Mortality
As in prior studies, extraction of cardiac implanted electronic devices is underused. “This is a problem,” Jonathan Piccini says.
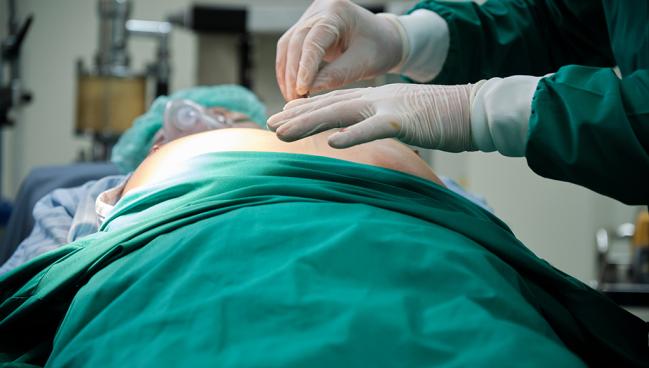
The vast majority of Medicare beneficiaries with infection of a cardiac implanted electronic device (CIED) don’t have their hardware removed within 30 days, contrary to guideline recommendations, and that appears to be harming patient outcomes, according to a large study.
Overall, 18.6% of patients with a CIED infection had the device and leads removed within 30 days of diagnosis, and this was associated with a lower rate of all-cause mortality through follow-up, particularly if extraction occurred within the first 6 days, lead author Sean Pokorney, MD (Duke University Medical Center, Durham, NC), and colleagues report.
The findings, published online this week in JAMA Cardiology, are consistent with those from prior studies showing both low rates of extraction and worse outcomes among patients whose hardware isn’t removed. Of note, based on data showing that antibiotic therapy alone is not enough to contend with CIED infections, practice guidelines provide a class I indication for complete removal of the device and leads in this scenario.
“This is a problem that we all need to think a lot more about,” senior author Jonathan Piccini, MD (Duke University Medical Center), told TCTMD. “Every healthcare member of the team is doing their best, but the question is how can the system do better for the patients that have device infection, and that’s what we need to focus on.”
Suboptimal Extraction Rates
For the study, the investigators examined data on about 1.07 million fee-for-service Medicare beneficiaries who had received a pacemaker, implantable cardioverter-defibrillator (ICD), or cardiac resynchronization therapy (CRT) device. Overall, 1.1% of patients had a CIED infection—defined as endocarditis or infection of the device implant accompanied by documented antibiotic therapy within 30 days of diagnosis—at least a year after implantation (mean 3.7 years). The median age of affected patients was 75, and 60.1% were men.
Most patients with a CIED infection did not undergo extraction within 30 days of diagnosis—only 13.4% had their hardware removed within the first 6 days and another 5.2% underwent extraction 7 to 30 days after diagnosis. The rate of extraction reached 25.4% by 5 years.
Black individuals and women were less likely to have their hardware removed in response to a CIED infection, as were patients with diabetes, kidney disease, a history or stroke/TIA, and frailty.
This is a problem that we all need to think a lot more about. Jonathan Piccini
Cumulative 1-year mortality varied based on extraction status, ranging from a low of 18.6% among patients who underwent extraction within 6 days to a high of 32.5% among those who didn’t have their hardware removed; the rate was 23.4% among those who underwent extraction 7 to 30 days after diagnosis.
Any extraction was associated with a lower risk of mortality compared with no extraction (adjusted HR 0.82; 95% CI 0.74-0.90), with an even stronger relationship when the procedure was performed within 6 days (adjusted HR 0.69; 95% CI 0.61-0.78). Extraction 7 to 30 days after diagnosis was not significantly associated with a reduced mortality risk (adjusted HR 0.88; 95% CI 0.73-1.04).
What’s Standing in the Way
As for why extraction rates are so low despite strong recommendations for the practice in guidelines, Piccini pointed to multiple factors. Not all centers provide the service and not all physicians who care for the types of patients who develop CIED infections may be thinking about infection as a possibility when evaluating symptoms.
Quality-improvement initiatives, such as one called RECTIFY, and efforts to better identify affected patients—with alerts within the electronic medical record in response to information indicative of a possible CIED infection, for instance—might help bump extraction rates higher, Piccini suggested. He noted, too, that the American Heart Association has started a National CIED Infection Initiative to raise awareness of the problem.
And to improve access to extraction, there might be a need for centers of excellence focused on the issue, much like what is used in the realm of orthopedic prosthesis infections, he suggested.
“All of those things are needed to try and help us get on top of improving our care for these patients who are at a very vulnerable period,” Piccini said. “Having a device infection has very serious implications for someone’s future health.”
Jim Cheung, MD (Weill Cornell Medicine, New York, NY), has looked into the use of lead extraction for device-related endocarditis, finding an even lower rate of extraction than what was observed in the current study, which affirms that “our treatment of infections associated with cardiac implantable devices remains suboptimal.”
He also pointed to multiple potential reasons for that, including a misperception among some physicians that antibiotics are enough, concerns about the risks of extraction, inadequate access to experienced extraction centers, and—in some cases—legitimate medical reasons to avoid the procedure.
To help boost numbers, Cheung said “there needs to be physician education” to raise awareness of device infections and appropriate management, as well as education of patients, who might not develop an infection until many years after implantation, about what to look out for.
With multiple studies addressing this issue, “I think that finally we are getting a wealth of nationally representative data that is identifying the scope of the problem,” Cheung said. “Now that we’ve shown a little bit of the first step of identifying the scope of the problem, it behooves us to implement an action plan that allows us to actually address the problem.”
Todd Neale is the Associate News Editor for TCTMD and a Senior Medical Journalist. He got his start in journalism at …
Read Full BioSources
patients with cardiac implanted electronic device infection.]( https://jamanetwork.com/journals/jamacardiology/fullarticle/2810389) JAMA Cardiol. 2023;Epub ahead of print.
Disclosures
- The study was funded by a grant from Philips to the Department of Population Health at Duke University.
- Pokorney reports receiving grants from Philips during the conduct of the study; personal fees from Philips, Boston Scientific, Medtronic, Milestone Pharmaceuticals, Zoll, Bristol Myers Squibb, Pfizer, and Sanofi outside the submitted work; and grants from Boston Scientific, Medtronic, Bristol Myers Squibb, Pfizer, and Sanofi outside the submitted work.
- Piccini reports receiving personal fees from Philips and Medtronic for consulting and grants from Abbott and AAMI outside the submitted work.



Comments